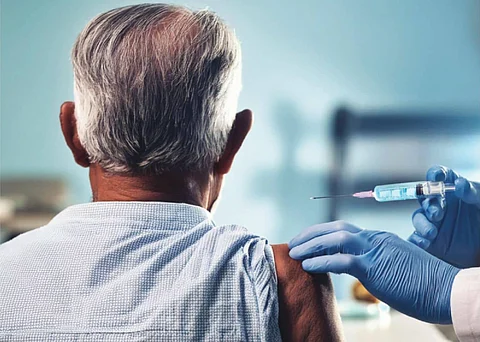

On a humid July morning, 20-year-old Gaurav Joshi walked inside a polyclinic in northeast Delhi for tuberculosis (TB) vaccination.
Joshi’s father has TB. A few weeks earlier, an Accredited Social Health Activist (ASHA), on a visit to their house for a survey, had informed Joshi that he needed to get revaccinated to reduce his chances of contracting the disease.
A poster welcomed him: ‘BCG ka teeka ek surakshit teeka hai (BCG vaccine is a safe vaccine)’, before he entered the immunisation room.
The auxiliary nurse midwife (ANM) on duty wrote his details on a register. She took about 10-15 seconds to quickly narrate the purpose of the vaccine, from a long information sheet, containing 10 points about the ‘research study’ on adult Bacille Calmette-Guerin (BCG) vaccination. The points talked about the vaccine’s side effects and precautions to be taken, among other things.
Joshi was asked to sign against his name; which he does, without reading. This now makes him an unwitting participant of a ‘programmatic research implementation study’ on TB BCG vaccination targeting high-risk adults.
The vaccine administered to him is the same one that he had received as an infant under India’s Universal Immunisation Programme. In about three to four minutes that the process lasted, Joshi successfully became a “beneficiary” of the programme.
At some distance from the polyclinic, inside a dimly lit hall that serves as an anganwadi, a discussion among four ASHA workers about the consequences they will be facing “if something goes wrong” gave a glimpse into the current concerns.
ASHAs are community health activists who act as an interface between the community and the public health system and are tasked with creating awareness on health and mobilising the community towards local health planning, among other things.
In this case, ASHAs were asked to survey the eligible population and mobilise people for the vaccine. “We have to convince them that the vaccine is important and is safe, but we don’t know if it’s a trial or not and we are concerned that people will lose their trust in us if something goes wrong,” said a worker who did not want to be identified.
Another ASHA worker added that after the COVID-19 vaccination, people had been reporting different health problems and were apprehensive of recieving another vaccine.
Was it a vaccination drive or a study? The answer is not clear.
There are glaring problems either way one looks at it. If it is a vaccination drive, then the research to establish the success of a booster shot of BCG in adults for preventing tuberculosis is inconclusive.
And if it is a clinical trial to test the success of BCG in adults, then it lacks the rigour of the process — a four-phase drug test that can go on for years.
“Vaccines undergo clinical trials in the target age group before a rollout. Here, BCG is an already approved vaccine for a certain age group, being given to a different age group not after a conventional trial but in a programme implementation study mode under routine services,” Swathi Krishna, former national consultant at central TB division, told Down To Earth (DTE).
India accounts for 27 per cent of the world’s TB cases, as per Global Tuberculosis Report 2023 by the World Health Organization (WHO). Pulmonary TB (or TB of the lungs) is the most common and contagious form of the disease. The other forms of TB result from the spread of the infection from the lungs to other organs.
It is the penultimate year of the central government’s rather implausible goal to end TB by 2025 — five years ahead of the deadline set under the United Nations-mandated Sustainable Development Goals (SDG). The adult vaccination programme seems a desperate attempt to reach that goal.
In this rush to achieve numbers, till August 2024, over 2.5 million people, including those older than 60 years and with diseases like diabetes across different states, are being revaccinated with this live accentuated BCG vaccine, essentially a vaccine for neonates.
The revaccination programme started last year, when the Union ministry of health and family welfare (MOHFW) decided to “conduct a programme implementation research study of BCG vaccination in adults” and sought states’ consent to participate in it, as per Standard Operating Procedures (SOP) for Adult BCG Vaccination under programmatic implementation study in India — a document not available on the ministry’s website but in circulation among health officials and medical professionals, one of whom provided it to DTE. A total of 23 states and Union territories agreed to participate in the study.
To identify people who should be vaccinated, the government conducted surveys in March-April through local health workers such as ASHAs and ANMs. Any individual over 18 years of age, who has a history of the disease or has been exposed to it, is eligible for the booster dose, said the SOP.
Individuals aged over 18 years fitting into any of these six criteria and willing to take the vaccine are eligible for the adult tuberculosis (TB) vaccination:
History of TB disease: People who have had at least one episode of TB in the past five years.
Close contacts of TB patients: This will include contacts of current TB patients as well as all those contacts of index TB cases enrolled on Ni-kshay portal from January 1, 2021.
Individuals with a body mass index of less than 18 kg/sqm.
Individuals aged 60 years or above.
Individuals with a history of smoking tobacco (current user/past user, self reported).
Individuals with a history of diabetes (self reported).
In the 23 states and UTs, MOHFW has selected 547 districts for the programme. Of these, 274 districts are in the “intervention” arm, while 273 districts are in the “comparator” arm. The vaccinated will be followed up for an analysis for up to three years. Data from the comparison of the two arms will be used to assess the success of the programme, stated the SOP.
The vaccination has started in May and is going on in Delhi, Himachal Pradesh and Assam, while preparations are underway in Maharashtra.
“Since the vaccine is already licenced for use on children, there is no need for time-consuming trials. This is the rationale behind the programme research study,” said an MOHFW official, requesting anonymity.
If that is the case, then the SOP should not seek “to determine the effectiveness of adult BCG vaccination” and “to determine safety of adult BCG vaccination under programmatic settings in vaccinated individuals with annual review”.
Experts said that the stringent guidelines that a trial has to follow are surpassed during a programme. The foremost being the controlled settings in which a trial is done.
Take, for instance, 42-year-old Anita’s (name changed) family. She and her 20-year-old daughter Anjali (name changed) have recently been revaccinated, because the family, residing in Delhi, has been identified as ‘close contacts’ of a TB patient. When DTE spoke to her, Anita said her husband will also be getting the vaccination soon.
But nobody in the family has been diagnosed with TB.
Anita’s 17-year-old son met with an accident two years ago, which caused a lung injury and even though he was not diagnosed with TB, he was given TB medications to prevent any further infection.
When the ASHA, who had been asked to conduct a survey, came to identify people for revaccination and asked if there was any TB patient in the house, the family told about their son’s treatment and that is how they were identified as “close contacts” of a TB patient and were suggested to get the BCG vaccine, which they agreed to.
This callous approach of ‘self-reporting’ belies the meticulousness of a trial.
It has not been thought through properly. You do not give BCG to an adult without first administering a Mantoux skin test to detect prior exposure to TB. It is okay to give it to a child, as they have not had any exposure
Jacob Puliyel, paeditrician, Kochi.
The SOP only mentions that “wherever feasible, documentary evidence of diagnosis and treatment for diabetes will be obtained”.
“This is how a programme gets trivialised. Suppose you give this vaccine to people who have some disease and they are not aware of it or they have low levels of symptoms. In that case, nobody knows what harm the vaccine will cause,” said Yogesh Jain, public health physician based in Surguja, Chhattisgarh. “This is being given to the elderly population and to diabetics, who already have their immunity compromised.
BCG vaccine is known to have certain significant side effects in a small but definite proportion of people. “Basically, you are putting a live vaccine inside someone’s body. This means that the bacteria goes into and multiplies inside the body. But protocols are missing and nobody is checking these things,” said Pune-based public health expert Abhay Shukla, who is also the national co-convenor of Jan Swasthya Abhiyan.
Soumya Swaminathan, principal advisor for National Tuberculosis Elimination Programme, told DTE that the programme is more of an intervention.
“It is more of an intervention, a research implementation
study. Yes, there is not enough evidence that it is going to reduce incidence of tuberculosis (TB). When the ministry started this, the feeling among scientists was that this should be done as an implementation research study. So there are districts where the BCG vaccine is being rolled out to vulnerable groups and control districts. I do not think that intervention is something that can be scaled or should be scaled without further data and information. I hope that this exercise can really be used to generate that data. So this could be one of the largest studies of this kind,” Swaminathan said.
A January 2024 article in The Lancet, What is next for BCG revaccination to prevent tuberculosis?, said that if the revaccination does not prevent disease, then besides being a substantial waste of resources, it would expose large numbers of people to potential adverse effects, including the threat of disseminated disease in individuals with untreated HIV.
The plan to compare the “intervention” arm and the “comparator” arm also seems unclear. “In the ‘intervention’ arm, there will be a survey where people will be asked if they have TB symptoms. But in the ‘comparator’ arm, we do not plan a similar follow-up. The routine services under TB elimination programme will continue there,” T Ramesh, state TB officer, Andhra Pradesh TB control division, told DTE.
How, then, will the comparison be accurate?
Vaccination in a programme mode also bypasses any ethical review. Though the SOP mentioned that the programme has received an “ethics approval by ICMR [Indian Council of Medical Research]-CEHR [Central Ethics Committee on Human Research]”, a member of CEHR told DTE that it is out of the scope of the mandate of the committee to approve or disapprove any public health programme.
“This should be the mandate of the health ministry but it does not have an ethics committee and CECHR recommended to the ministry that it should have an ethics board. Typically, public health programmes do not need any ethical approvals,” the member said. During a discussion on the programme between the approval board members and MOHFW a few months ago, the former were told that the programme has not gone through an ethical approval, said the official.
Globally, BCG is the only clinically approved and licensed TB vaccine to be given at birth and has been used for more than a century. Its efficacy lasts for 10-15 years. In 2017, who advised against BCG revaccination in adults without further study, while recent studies on clinical trials in different nations have not found much protective effect in adults.
In Malawi, a randomised, placebo-controlled trial of BCG revaccination on more than 46,000 participants aged three months to 70 years found no significant overall protection against confirmed TB infection after six to nine years of follow-up.
A trial in Brazil in children aged 7-14 years, who had received one BCG vaccination as infants, concluded in 2005 that it does not provide substantial additional protection and should not be recommended. India’s own experience of revaccination by ICMR in Tamil Nadu’s Chengalpattu district between 1968 and 1983, in people aged one month or above, found no overall protection in adults and a low level of protection in children after a 15-year follow up period.
A retrospective analysis of this community trial published in 2022 found that BCG revaccination offered only 36 per cent protection against development of TB.
Revaccination with BCG is being done even as new vaccines for TB are under development. Since 2019, ICMR has been testing a recombinant BCG vaccine candidate (VPM1002) and a heat-killed suspension mycobacterium vaccine (Immuvac).
In March, Hyderabad-based Bharat Biotech International Ltd, in collaboration with Spain-based Biofabri, started a clinical trial in adults to evaluate the safety, immunogenicity and efficacy of mtbvac — the first TB vaccine derived from a human source. The vaccine is said to be more effective and longer-lasting than BCG in preventing TB in adults and adolescents.
“Why are we spending crores of rupees on revaccination from a vaccine that is going to be obsolete?” asked Gayatri Sharma, a public health professional based in Mumbai.
This was first published in the 1-15 September, 2024 Print edition of Down To Earth