
Chalmers University team use AI to model halide perovskites at unprecedented scale
Findings resolve long-standing mystery around formamidinium lead iodide
Work confirmed by experiments at University of Birmingham
Results published in Journal of the American Chemical Society
Discovery could advance durable solar technology for buildings, devices and more
Global electricity demand is climbing at an unprecedented pace and meeting it sustainably will depend on breakthroughs in renewable energy. Researchers at Chalmers University of Technology in Sweden say they have made a significant step forward in understanding halide perovskites, one of the most promising yet unstable materials for solar cells, using powerful computer simulations enhanced with machine learning.
The findings, published in the Journal of the American Chemical Society, could accelerate the development of more durable, high-efficiency solar cells that are thin and flexible enough to coat everything from smartphones to skyscrapers. They also demonstrate how artificial intelligence is transforming materials science by enabling researchers to solve problems once thought impossible.
According to the International Energy Agency, electricity’s share of global energy consumption is expected to surpass 50 per cent within the next 25 years, up from around 20 per cent today. That growth underscores the urgency of developing efficient, flexible and environmentally friendly energy conversion methods.
There is a significant and growing need for new, environmentally friendly and efficient energy conversion methods, such as more efficient solar cells, according to Julia Wiktor, associate professor at Chalmers and principal investigator of the study. “Our findings are essential to engineer and control one of the most promising solar cell materials for optimal utilisation. It’s very exciting that we now have simulation methods that can answer questions that were unresolved just a few years ago,” she said in a statement.
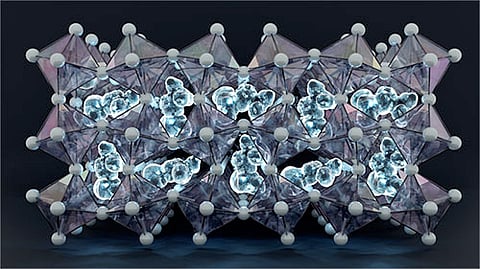
Halide perovskites are regarded as strong candidates for low-cost, lightweight and flexible solar cells, as well as devices such as LED (light emitting diode) bulbs, thanks to their ability to absorb and emit light with exceptional efficiency. But their instability has stalled wider use. A crystalline compound called formamidinium lead iodide shows particularly attractive properties, yet degrades too quickly to be practical.
The Chalmers team has now described in detail the low-temperature phase of this compound — a fundamental question that had eluded researchers for years. “The low-temperature phase of this material has long been a missing piece of the research puzzle and we’ve now settled a fundamental question about the structure of this phase,” said Sangita Dutta, researcher at Chalmers.
Modelling perovskites has been notoriously difficult because capturing their properties accurately requires immense computing power. By combining traditional methods with machine learning, the researchers extended their simulations thousands of times longer than before and scaled up models from hundreds of atoms to millions. This allowed them to capture behaviour closer to real-world conditions.
The simulations showed that as the material cools, formamidinium molecules become stuck in a semi-stable state. To validate their models, the team collaborated with experimental scientists at the University of Birmingham, who cooled the compound to -200 degrees Celsius (°C) and confirmed the results in the lab.
“We hope the insights we’ve gained from the simulations can contribute to how to model and analyse complex halide perovskite materials in the future,” said Erik Fransson of Chalmers’ Department of Physics.